1. What is a clinical trial?
A clinical trial is a carefully designed study which tests the benefits and risks of a specific medical treatment or intervention, such as a new drug. Every life-saving or life-enhancing medicine that is helping patients today once was the focus of a clinical trial.
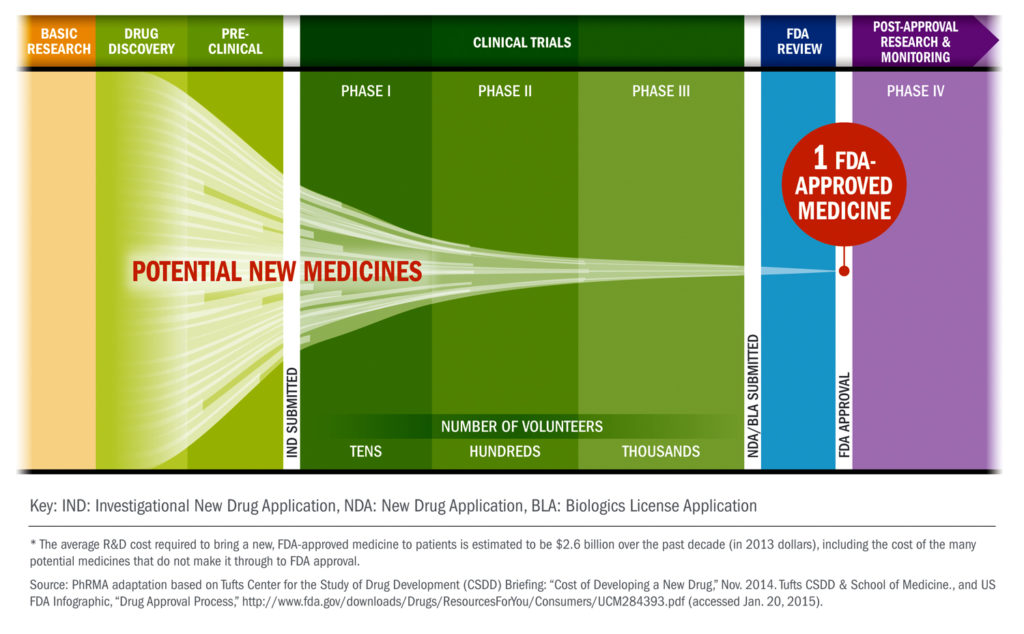
2. Drugs go through a long journey before getting approved by the FDA.
From drug discovery through FDA approval, developing a new medicine takes at least 10 years on average and costs an average of $2.6 billion. Less than 12% of the candidate medicines that make it into Phase 1 clinical trials will be approved by the FDA. Less than 12% of the candidate medicines that make it into Phase 1 clinical trials will be approved by the FDA.
3. There are currently 257,799 research studies in all 50 states and in 201 countries listed on clinicaltrials.gov.
ClinicalTrials.gov is a Web-based resource that provides patients, their family members, health care professionals, researchers, and the public with easy access to information on publicly and privately supported clinical studies on a wide range of diseases and conditions. The Web site is maintained by the National Library of Medicine (NLM) at the National Institutes of Health (NIH).
4. What is a placebo?
A placebo is an inactive pill, liquid, or powder that has no treatment value. In clinical trials, experimental treatments are compared with placebos to assess the treatment’s effectiveness. In some studies, the participants in the control group will receive a placebo instead of an active drug or treatment.
5. Not every clinical trial involves investigational medication.
Different types of clinical trials include:
- Treatment trials – test new treatments, new drug combinations, or new approaches to surgery or therapy.
- Prevention trials – search for better ways to prevent disease. Approaches may include medicines, vitamins, vaccines, minerals, or lifestyle changes.
- Diagnostic trials – conducted to find better tests or procedures for diagnosing a particular disease or condition.
- Screening trials – test the best way to detect certain diseases or health conditions.
- Quality of Life trials – explore ways to improve the comfort or quality of life for individuals with a chronic illness.
Sources: PhRMA, Coalition for Clinical Trials Awareness, CISCRP
